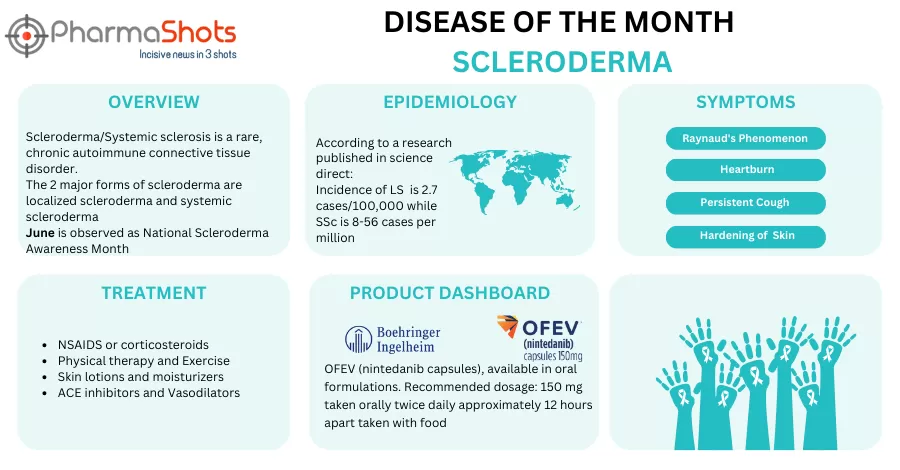
Cabaletta Bio’s CABA-201 Receives the US FDA’s Orphan Drug Designation to Treat Systemic Sclerosis
Shots:
- The US FDA has granted ODD to the company’s CABA-201 for treating systemic sclerosis (SSc). CABA-201 is being evaluated under a series of P-I/II (RESET) studies, incl. RESET-SSc, for addressing various autoimmune diseases
- The P-I/II (RESET-SSc) trial assesses CABA-201 (1 x 10^6 cells/kg, single infusion) to treat SSc across 2 arms, severe skin arm for patients (n=6) with severe skin involvement & the organ arm for patients (n=6) meeting the pulmonary, cardiac, or renal involvement criteria irrespective of skin involvement
- CABA-201 is developed to decrease CD19+ B cells after a single infusion enabling an immune system reset for durable remission off therapy in patients with autoimmune diseases
Ref: Cabaletta | Image: Cabaletta
Related News:- UCB Receive the US FDA’s Approval of Zilbrysq (zilucoplan) for the Treatment of Adults with Generalized Myasthenia Gravis
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.